Industrial electrocoating is a widely used surface treatment for protecting the metal castings and CNC machining products from the corrosion with nice finish. Many customers ask questions about the surface treatment of metal castings and precision machined parts. This article will focus on the electrophoretic coating process. Hope it will be helpful to all partners.
Electrocoating is a coating method in which particles such as pigments and resins suspended in the electrophoretic solution are oriented to migrate and deposit on the surface of one of the electrodes by using an external electric field. The principle of electrophoretic coating was invented at the end of the 1930s, but this technology was developed and obtained industrial application after 1963. Electrophoretic coating is the most practical construction process for water-based coatings. Electrophoretic coating has the characteristics of water solubility, non-toxicity, and easy automatic control. Because it is suitable for the surface treatment of conductive workpieces (metal castings, machined parts, forgings, sheet metal parts and welding parts, etc.), the electrophoretic coating process has quickly been widely used in industries such as automobiles, building materials, hardware, and home appliances.
Principles
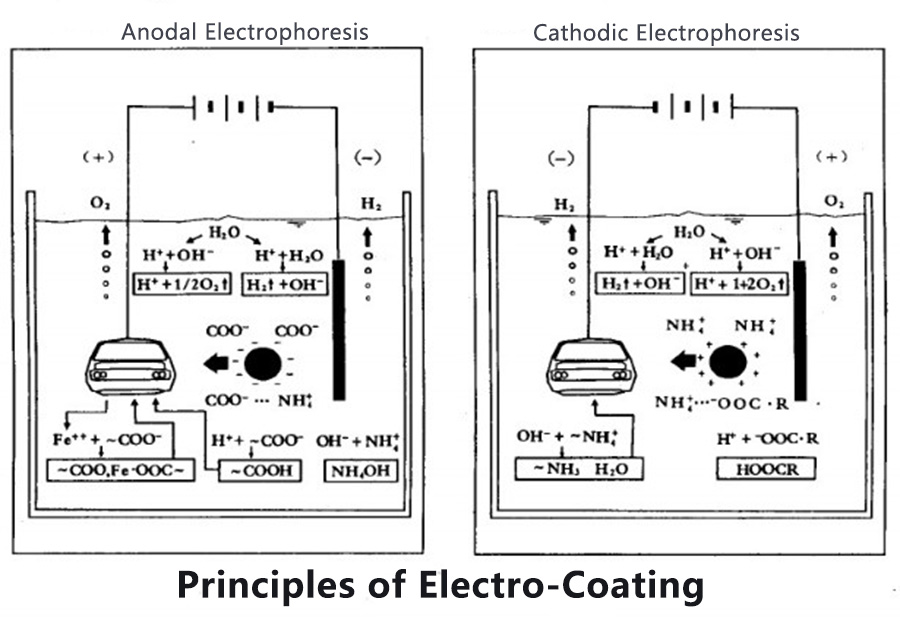
The resin contained in the cathodic electrophoretic coating has basic groups, which form a salt after acid neutralization and dissolve in water. After the direct current is applied, the acid radical negative ions move to the anode, and the resin ions and the pigment particles wrapped by them move to the cathode with positive charges and are deposited on the cathode. This is the basic principle of electrophoretic coating (commonly known as plating). Electrophoresis coating is a very complex electrochemical reaction, at least four effects of electrophoresis, electrodeposition, electrolysis, and electroosmosis occur simultaneously.
Electrophoresis
After the anode and cathode in the colloidal solution are powered on, the colloidal particles move to the cathode (or anode) side under the action of the electric field, which is called electrophoresis. The substance in the colloidal solution is not in the state of molecules and ions, but the solute dispersed in the liquid. The substance is large and will not precipitate into a dispersed state.
Electrodeposition
The phenomenon of solid precipitation from liquid is called agglomeration (agglomeration, deposition), which is generally produced when cooling or concentrating the solution, and electrophoretic coating relies on electricity. In cathodic electrophoretic coating, positively charged particles aggregate on the cathode, and negatively charged particles (ie ions) aggregate on the anode. When the positively charged colloidal particles (resin and pigment) reach the cathode (substrate) After the surface area (highly alkaline interface layer), electrons are obtained and react with hydroxide ions to become water-insoluble substances, which are deposited on the cathode (painted workpiece).
Electrolysis
In a solution with ionic conductivity, the anode and cathode are connected to direct current, anions are attracted to the anode, and cations are attracted to the cathode, and a chemical reaction occurs. The anode produces metal dissolution and electrolytic oxidation to produce oxygen, chlorine, etc. The anode is an electrode that can produce an oxidation reaction. The metal is precipitated at the cathode and the H+ is electrolytically reduced to hydrogen.
Electroosmosis
After the two ends (cathode and anode) of solutions with different concentrations separated by a semipermeable membrane are energized, the phenomenon that the low-concentration solution moves to the high-concentration side is called electroosmosis. The coating film just deposited on the surface of the coated object is a semi-permeable film. Under the continuous action of the electric field, the water contained in the smearing film dialysis out of the film and moves to the bath to dehydrate the film. This is electroosmosis. Electroosmosis turns the hydrophilic coating film into a hydrophobic coating film, and dehydration makes the coating film dense. The wet paint after swimming with good electro-osmosis electrophoretic paint can be touched and not sticky. You can rinse off the bath liquid adhering to the wet paint film with water.
Characteristics of Electrocoating
Electrophoretic paint film has the advantages of fullness, uniformity, flatness and smooth coating. The hardness, adhesion, corrosion resistance, impact performance, and permeability of electrophoretic paint film are significantly better than other coating processes.
(1) Water-soluble paint is used, water is used as the dissolving medium, which saves a lot of organic solvents, greatly reduces air pollution and environmental hazards, is safe and sanitary, and avoids the hidden danger of fire;
(2) The painting efficiency is high, the paint loss is small, and the utilization rate of the paint can reach 90% to 95%;
(3) The coating film thickness is uniform, the adhesion is strong, and the coating quality is good. Each part of the workpiece, such as the inner layer, depressions, welds, etc., can obtain a uniform and smooth coating film, which solves the problem of other coating methods for complex-shaped workpieces. The painting problem;
(4) The production efficiency is high, and the construction can realize automatic and continuous production, which greatly improves labor efficiency;
(5) The equipment is complex, the investment cost is high, the power consumption is large, the temperature required for drying and curing is high, the management of paint and painting is complicated, the construction conditions are strict, and wastewater treatment is required;
(6) Only water-soluble paint can be used, and the color cannot be changed during the coating process. The stability of the paint is not easy to control after storage for a long time.
(7) The electrophoretic coating equipment is complicated and the technology content is high, which is suitable for the production of fixed color.
Limitations of Electrocoating
(1) It is only suitable for primer coating of conductive substrates such as machinery parts of ferrous metals and non-ferrous metals. Non-conductive objects such as wood, plastic, cloth, etc. cannot be coated with this method.
(2) Electrophoretic coating process is not suitable for the coated objects composed of multiple metals, if the electrophoresis characteristics are different.
(3) Electrophoretic coating process cannot be used for the coated objects that cannot withstand high temperature.
(4) Electrophoretic coating is not suitable for coating with limited requirements on color. Electrophoretic coating of different colors needs to be painted in different grooves.
(5) Electrophoretic coating is not recommended for small-batch production (the renewal period of the bath is more than 6 months), because the renewal speed of the bath is too slow, the resin in the bath is aging and the solvent content changes greatly. The bath is unstable.
Steps of Electrocoating
(1) For electrophoretic coating of general metal surfaces, the process flow is: pre-cleaning → degreasing → water washing → rust removal → water washing → neutralization → water washing → phosphating → water washing → passivation → electrophoretic coating → tank top Cleaning → ultrafiltration water washing → drying → offline.
(2) The substrate and pretreatment of the coated object have a great influence on the electrophoretic coating film. Metal castings are generally derusted by sandblasting or shot blasting, cotton yarn is used to remove floating dust on the surface of the workpiece, and sandpaper is used to remove residual steel shots and other debris on the surface. The steel surface is treated with degreasing and rust removal. When the surface requirements are too high, phosphating and passivation surface treatments are required. Ferrous metal workpieces must be phosphated before anodic electrophoresis, otherwise the corrosion resistance of the paint film will be poor. In phosphating treatment, zinc salt phosphating film is generally selected, with a thickness of about 1 to 2 μm, and the phosphate film is required to have fine and uniform crystals.
(3) In the filtration system, the primary filtration is generally adopted, and the filter is a mesh bag structure. The electrophoretic paint is transported to the filter through a vertical pump for filtration. Considering the comprehensive replacement cycle and the quality of the paint film, the filter bag with a pore size of 50μm is the best. It can not only meet the quality requirements of the paint film, but also solve the problem of filter bag clogging.
(4) The size of the circulation system of electrophoretic coating directly affects the stability of the bath and the quality of the paint film. Increasing the circulation volume reduces the precipitation and bubbles of the bath liquid; however, the aging of the bath liquid accelerates, the energy consumption increases, and the stability of the bath liquid becomes worse. It is ideal to control the cycle times of the tank liquid to 6-8 times/h, which not only guarantees the quality of the paint film, but also ensures the stable operation of the tank liquid.
(5) As the production time increases, the impedance of the anode diaphragm will increase and the effective working voltage will decrease. Therefore, in production, the operating voltage of the power supply should be gradually increased according to the voltage loss to compensate for the voltage drop of the anode diaphragm.
(6) The ultrafiltration system controls the concentration of impurity ions brought by the workpiece to ensure the quality of coating. In the operation of this system, it should be noted that once the system is in operation, it should run continuously and it is strictly prohibited to run intermittently to prevent the ultrafiltration membrane from drying up. The dried resin and pigment adhere to the ultrafiltration membrane and cannot be cleaned thoroughly, which will seriously affect the water permeability and service life of the ultrafiltration membrane. The water output rate of the ultrafiltration membrane shows a downward trend with the running time. It should be cleaned once for 30-40 days of continuous work to ensure the ultrafiltration water required for ultrafiltration leaching and washing.
(7) The electrophoretic coating method is suitable for the production process of a large number of assembly lines. The renewal cycle of the electrophoresis bath should be within 3 months. The scientific management of the bath is extremely important. Various parameters of the bath are regularly tested, and the bath is adjusted and replaced according to the test results. Generally, the parameters of the bath solution are measured at the following frequency: the pH value, solid content and conductivity of the electrophoresis solution, ultrafiltration solution and ultrafiltration cleaning solution, anion (anode) polar solution, circulating lotion, and deionization cleaning solution once a day; Base ratio, organic solvent content, and laboratory small tank test twice a week.
(8) For the management of the quality of the paint film, the uniformity and thickness of the paint film should be checked frequently, and the appearance should not have pinholes, sagging, orange peel, wrinkles, etc. Regularly check the physical and chemical indicators such as the adhesion and corrosion resistance of the coating film. The inspection cycle is in accordance with the manufacturer's inspection standards, and generally each batch needs to be inspected.
Surface Treatment Before Electrophoresis
The surface treatment of the workpiece before coating is an important part of electrophoretic coating, mainly involving degreasing, rust removal, surface conditioning, phosphating and other processes. The quality of its treatment not only affects the appearance of the film, reduces the anti-corrosion performance, but also destroys the stability of the paint solution. Therefore, for the surface of the workpiece before painting, it is required to be free of oil stains, rust marks, no pretreatment chemicals and phosphating sedimentation, etc., and the phosphating film has dense and uniform crystals. Regarding the various pre-treatment processes, we will not discuss them individually, but only put forward a few points of attention:
1) If the degreasing and rust are not clean, it will not only affect the formation of phosphating film, but also affect the bonding force, decorative performance and corrosion resistance of the coating. The paint film is prone to shrinkage and pinholes.
2) Phosphating: The purpose is to improve the adhesion and anti-corrosion ability of the electrophoretic film. Its role is as follows:
(1) Due to physical and chemical effects, the adhesion of the organic coating film to the substrate is enhanced.
(2) The phosphating film turns the metal surface from a good conductor to a poor conductor, thereby inhibiting the formation of micro-batteries on the metal surface, effectively preventing the corrosion of the coating, and increasing the corrosion resistance and water resistance of the coating. In addition, only on the basis of thorough bottoming and degreasing, a satisfactory phosphating film can be formed on a clean, uniform, and grease-free surface. From this aspect, the phosphating film itself is the most intuitive and reliable self-check on the effect of the pretreatment process.
3) Washing: The quality of washing at each stage of the pretreatment will have a great influence on the quality of the entire pretreatment and paint film. The last deionized water cleaning before painting, make sure that the dripping conductivity of the coated object is not greater than 30μs/cm. The cleaning is not clean, such as the workpiece:
(1) Residual acid, phosphating chemical liquid, flocculation of resin in paint liquid, and deterioration of stability;
(2) Residual foreign matter (oil stains, dust), shrinkage holes, particles and other defects in the paint film;
(3) Residual electrolytes and salts lead to aggravation of electrolysis reaction and produce pinholes and other maladies.
Post time: Apr-17-2021